NEWS / EVENTS
LABone Participates in the 2025 Proficiency Testing Program Summary Conference on Interlaboratory Comparison
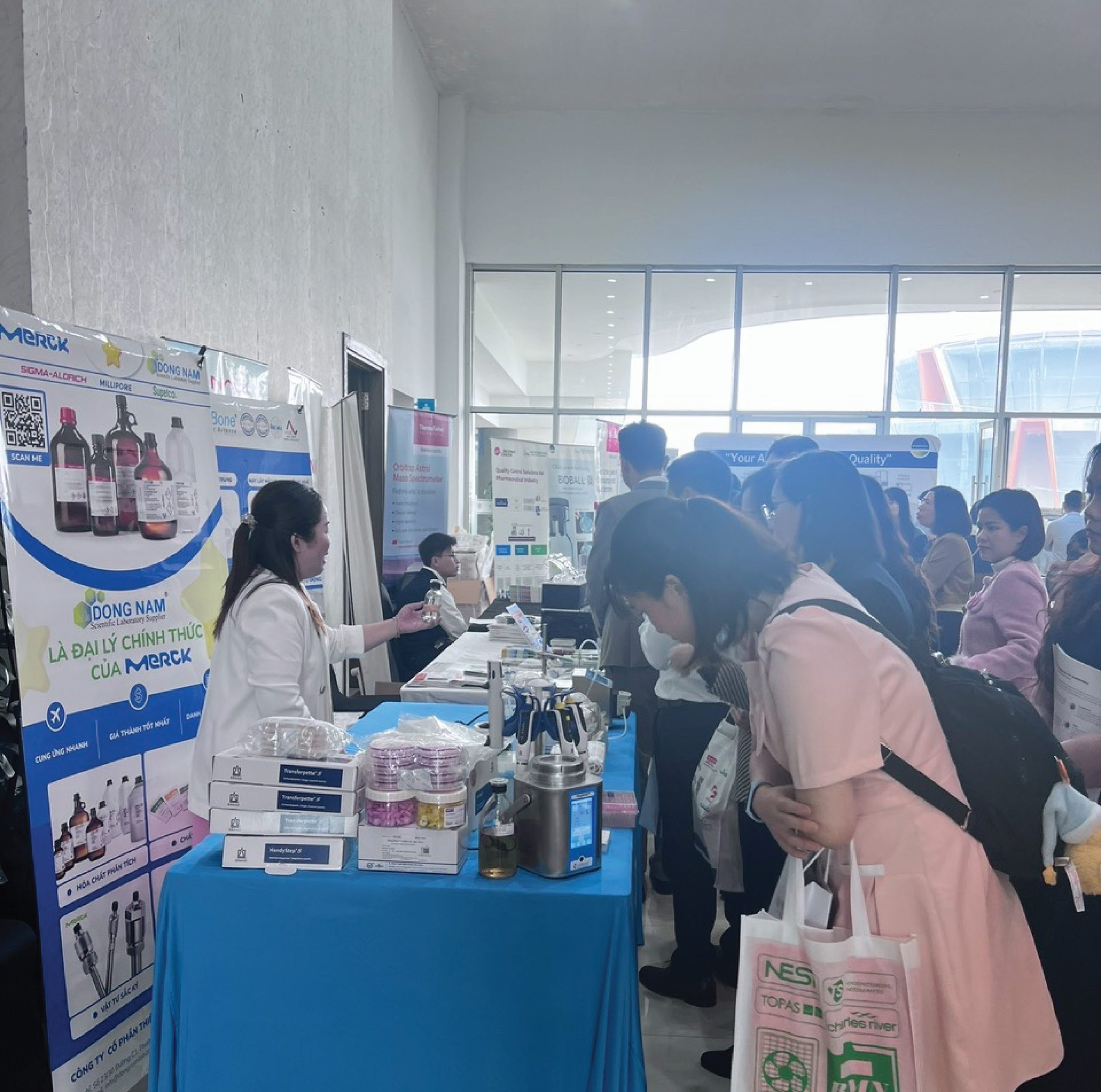
On December 12, 2025, LABone Science Equipment Co., Ltd. was honored to participate in and showcase its products at the conference “Summary of the 2025 Proficiency Testing Program through Interlaboratory Comparison,” organized by the National Institute of Drug Quality Control (NIDQC) at The Reed Hotel, Ninh Binh.
The event brought together leaders of Departments and Institutes under the Ministry of Health, representatives from 34 provincial/municipal Drug Quality Control Centers, along with numerous enterprises in the pharmaceuticals and quality testing sectors. This was an important occasion to review proficiency testing activities, share technical experience, and update new technical solutions for the national quality control system.
LABone – Comprehensive Solutions Provider
At the event, LABone introduced a full range of microbiology solutions designed to meet the diverse needs of Drug Quality Control Centers and laboratories across the country—focusing on safety, accuracy, and optimized operational efficiency.
-
Highlights include pre-poured microbiological media plates with 3-layer packaging, sterility testing media bottles, airborne microbial samplers, media dispensing systems, and more. These products ensure stability, safety, and suitability for quality control units throughout storage, transportation, and use.
LABone’s solutions aim to support laboratories in improving quality, streamlining workflows, and meeting both national and international standards. -
All product lines manufactured and supplied by LABone are developed with an emphasis on stability – accuracy – safety, helping Drug Quality Control Centers strengthen testing capacity, optimize procedures, and meet increasingly stringent quality assurance requirements.
Affirming Our Role as a Trusted Partner of the Quality Control System
Participation in the conference provided LABone with the opportunity to:
-
Meet and understand the real-world needs of drug quality control units.
-
Present new technical solutions that enhance the quality control of pharmaceuticals, cosmetics, and food products.
-
Collaborate with the National Institute of Drug Quality Control in the shared goal of standardization, modernization, and international integration.
LABone sincerely thanks the Organizing Committee and all delegates for visiting our exhibition booth. We remain committed to continued research, development, and delivery of comprehensive solutions contributing to the sustainable advancement of Vietnam’s quality control sector.
LABone Science Equipment Co., Ltd.
Address: 228/13/3 Nguyen Thi Lang Street, Phu Loi Hamlet, Cu Chi District, Ho Chi Minh City, Vietnam
Hotline: 0978 782 147
Email: info@labone.vn
Tax Code: 0312088377
Website: labone.com.vn


 Vn
Vn